Automating a clinical workflow creates a stable and repeatable clinical analysis. Automation reduces the potential to introduce human error, helps in regulatory compliance, and improves the precision of the clinical results. It is important to know that if you run a sample through your clinical pipeline, you are going to get the same results today as you will in 6… Read more »
If we take a look back in time, a lot happened in September 1998. It is the month in which the first ever “Who wants to be a Millionaire?” show debuted on ITV in Britain. Larry Page and Sergey Brin incorporated Google in September, registering the Google.com domain on September 15, 1998. And on that very same day, we officially… Read more »
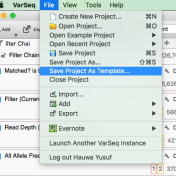
Question: Now that I’ve added annotation sources for my sample, filtered down to a list of interesting variants, flagged those variants and generated a clinical report, can I save or copy the annotation sources and filters for use on another sample? Short Answer: Yes! Long Answer: VarSeq was created with ease and efficiency in mind. In VarSeq, once you’ve defined… Read more »
Golden Helix in 2016 We had a terrific year 2015. It was the year in which we got serious about the clinical testing market. We successfully continued on the path of attracting more referenceable clients such as University of Iowa, Baby Genes, Prevention Genetics and many more. We rounded out our VarSeq suite by adding more clinically relevant features and… Read more »
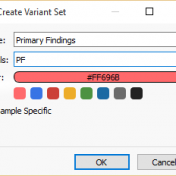
With the release of VSReports, we added the ability to “select” rows of your filtered output (often variants, but potentially things like coverage regions or genes) with a new feature dubbed “Record Sets”, but more often described as “colored checkboxes” for your tables. Although necessary for the important task of marking primary, secondary or other sets of variants for a… Read more »
The next release of VarSeq will ship a new product that is highly relevant to our customers in clinical testing labs. Via VSReports, VarSeq now has the ability to generate clinical-grade reports. These reports are fully customizable, containing focused and actionable data. VS Reports ships with report templates that are modeled off of the ACMG guidelines, the de-facto gold standard… Read more »
A prerequisite for clinical NGS interpretation is ensuring that the data being analyzed is of high enough quality to support the test results being returned to the physician. The keystone of this quality control process is coverage analysis. Coverage analysis has two distinct parts. Ensure that there is sufficient coverage to be confident in called variants Make certain that no… Read more »
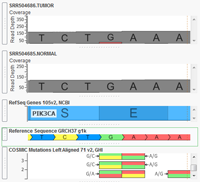
VarSeq now supports analysis of paired Tumor/Normal samples! Tumor/Normal support has been one of the most common feature requests for VarSeq since it was launched late last year, and we are excited to make this functionality available to all of our VarSeq users in the latest update (version 1.1.4). VarSeq is a powerful platform for annotation and filtering of DNA… Read more »