In addition to Golden Helix providing easy-to-use genomic software, we also provide value to our users by automating the curation of public databases used in our tools. These annotation sources serve multiple purposes not only leveraging key fields in the filter chain but also automatically supplying evidence for the ACMG and AMP classifications reviewed in VSClinical. Supporting the curation of… Read more »
With the release of VarSeq 2.2.4 just around the corner, I want to detail some new 2.2.4 features that will enhance somatic variant annotation and fusion analysis within VSClinical. A webcast back in July showed some of these updates in action, so if you are looking for some more content on this topic, I would highly recommend checking out the… Read more »
Golden Helix VSClinical provides a guided workflow interface for following the ACMG and AMP guidelines to evaluate variants and CNVs for NGS tests. The output of this work is most often a lab-specific clinical report. Since it was introduced, we have provided a powerful Word-based templating system to allow labs the ability to generate customized reports to include specific content… Read more »
Established in 2004 and headquartered in Chennai, India, with regional centers across the country, LifeCell runs India’s largest stem cell bank and has also diversified into diagnostics and tissue therapeutics. They employ roughly 2,000 people, providing genetic services to customers in the mother and baby space. Phani Nagaraja Setty is working as a scientist at LifeCell. Setty obtained his Master’s… Read more »
Welcome to the August 2021 edition of our customer publications blog post! Each month we spotlight a few recently published articles by our incredible Golden Helix customers. With users spanning both research and clinical spaces, the topics vary widely across many fields. This month, we will be highlighting VSClinical users and the guided workflow. Host Genetics and Antiviral Immune Responses… Read more »
In the July 2021 Customer Publication blog, I will highlight four studies that provided further insights into conditions that are typically identified in early childhood. As you will see as you read the summaries of each publication, both Golden Helix software platforms (VarSeq and SNP & Variation Suite or SVS for short) were instrumental in exploring the genetic factors that… Read more »
This blog post will cover an exciting new VSClinical feature in the upcoming VarSeq release. The ACMG Previously Interpreted Variants feature allows users to integrate databases of expert-curated variant interpretations into their VSClinical workflows. These data sources store variant-level interpretation data, including the classification, associated disorders, interpretation text, and scored criteria for each variant, along with notes providing a justification… Read more »
Could leveraging CNV analysis for whole exome or genome sequencing help provide answers for the undiagnosed? Roughly 4% of the world’s population shares one common desire – a diagnosis (1). Though it may seem counterintuitive, a diagnosis, no matter how grim, can provide relief, validation, the chance to make plans, and have a discernible sense of future for individuals and… Read more »
In this month’s customer publications blog, both of our flagship software platforms are shown hard at work to cover many scientific investigational topics. You will get a glimpse of how VSClinical can be used to dive deeper into a specific gene associated with breast cancer and how SVS is enabling scientific discoveries in Agrigenomics and human health and wellness. Read… Read more »
Dr. Auber is the team leader for the molecular genetic diagnosis of hereditary diseases at the Institute for Human Genetics at Hannover Medical School (MHH). MHH is one of the largest hospitals in northern Germany, with one of the largest outpatient clinics for individuals and families dealing with hereditary cancer and predisposition syndromes. Dr. Auber was in high school when… Read more »
Researchers and clinicians alike benefit from the powerful capabilities of Golden Helix’s software. Our tools are continuously validated, and we like to showcase a few articles each month that demonstrate the multitude of use cases and advancements in science. For our April 2021 customer publication installment, I would like to highlight the clinical space, with users spanning the globe, all… Read more »
With the latest release of VarSeq, we have made significant updates to our handling of the interaction of variants and genes. This includes the support for non-coding transcripts, improved splice site predictions, and updates to gene and transcript annotations. We received several questions regarding how decisions are made in the software regarding genes and transcripts with these gene-related changes. This… Read more »
Thank you to those who attended the recent webcast, “Exome Analysis with VS-CNV & VSClinical: Updated Strategies & Expanded Capabilities”. For those who could not attend but wish to watch, here is a link to the recording. In this webcast, we covered the capabilities and updates that have been incorporated into VarSeq that enhance whole exome sequencing workflows. The new… Read more »
In this blog, we will be covering new assessment catalogs and how they work to improve saving and tracking variant interpretations. VarSeq is a variant analysis tool that effectively analyzes single nucleotide (SNVs) and copy number variants (CNVs) in both cancer and germline workflows. Because VarSeq enables such diverse variant analysis, there are many research labs and institutions that evaluate… Read more »
Clinical testing labs produce reports as the end product of the NGS variant detection and interpretation workflow. Necessarily, the content, detail, and presentation of the report needs to be specialized to each clinical lab, and potentially each offered test. Our last blog post introduced the new Word-based report templates in VSClinical. In this blog post, we will introduce and explore… Read more »
New discoveries using NGS data analysis are never-ending and are pushing precision medicine to the forefront. In the January 2021 customer publication blog, I am focusing on our VarSeq software as investigators harness its power to perform a variety of investigational study designs. From cancer to inherited rare disease research, VarSeq is the rising star in research and diagnostic tools…. Read more »
Happy New Year! I hope you had an opportunity to relax over the holidays with your family and friends. It’s time to start talking about our plans for the New Year, but first, please allow me to review a few highlights from 2020 that helped build a foundation for our future. Growth: Golden Helix was named to the 2020 Inc… Read more »
Reading scientific articles that our customers have recently published is one of my favorite things here at Golden Helix. It is fascinating to learn about the research and to see the various ways our software gets put to the test.Since we are rolling out our most extensive VarSeq update yet, I thought it would be a great time to look… Read more »
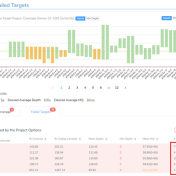
Curious about how coverage statistics can be used in conjunction with VarSeq? Evaluating the coverage over target regions or whole genomes is essential whether you are working with variant or CNV analysis. VarSeq has had the capability to compute sample level coverage statistics for some time now, but in the 2.2.2 release of VarSeq, there are some new features that… Read more »
Our team is regularly updating and curating annotations, most recently the BRCA Exchange. Breast cancer is known to occur in approximately 10% of the female population and if there is a damaging mutation in the breast cancer gene (BRCA), that rate increases to 65%. Although genetic testing can identify mutations in BRCA, a tumor suppressor gene, it has been difficult… Read more »