We have recently added a tutorial to help introduce customers to the ease and utility of the AMP Guidelines incorporated in VarSeq’s VSClinical package. The AMP Guidelines allow users to sort through available clinical evidence in a streamlined fashion to arrive at final classification and interpretation and then transfer that information into a clinical report. And the AMP Guidelines also incorporate the ACMG Guidelines capability!
What are the AMP Guidelines?
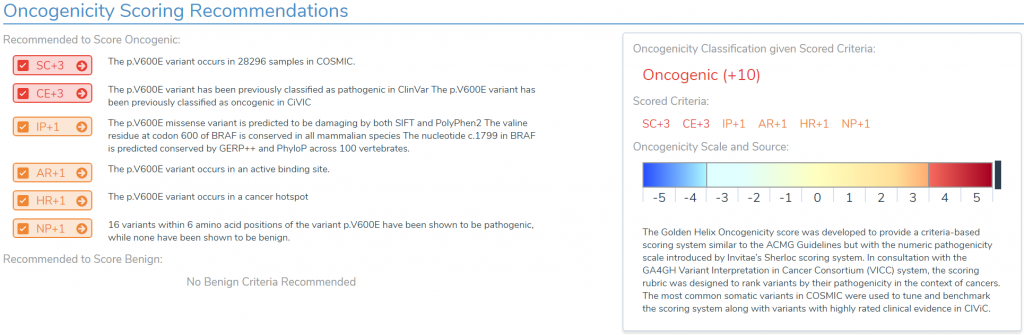
The Association for Molecular Pathology Guidelines (or AMP Guidelines) seek to define the oncogenicity of somatic biomarkers on a scale of Oncogenic to Benign (this differs from the standalone ACMG Guidelines analysis of germline variants, of which the tutorial can be found here). These biomarkers can include single nucleotide variants, insertions or deletions, copy number variants, gene fusions, and wild type genes. And the oncogenicity scoring incorporates factors such as previous classifications in sources like COSMIC, genetic locations relative to binding sites and hot spots, and possible nearby biomarker effects.
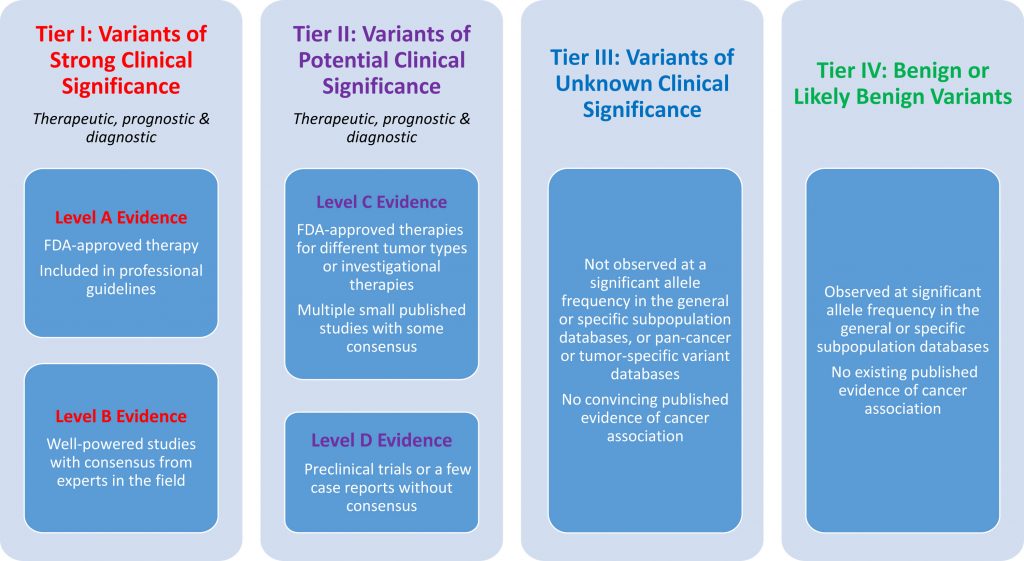
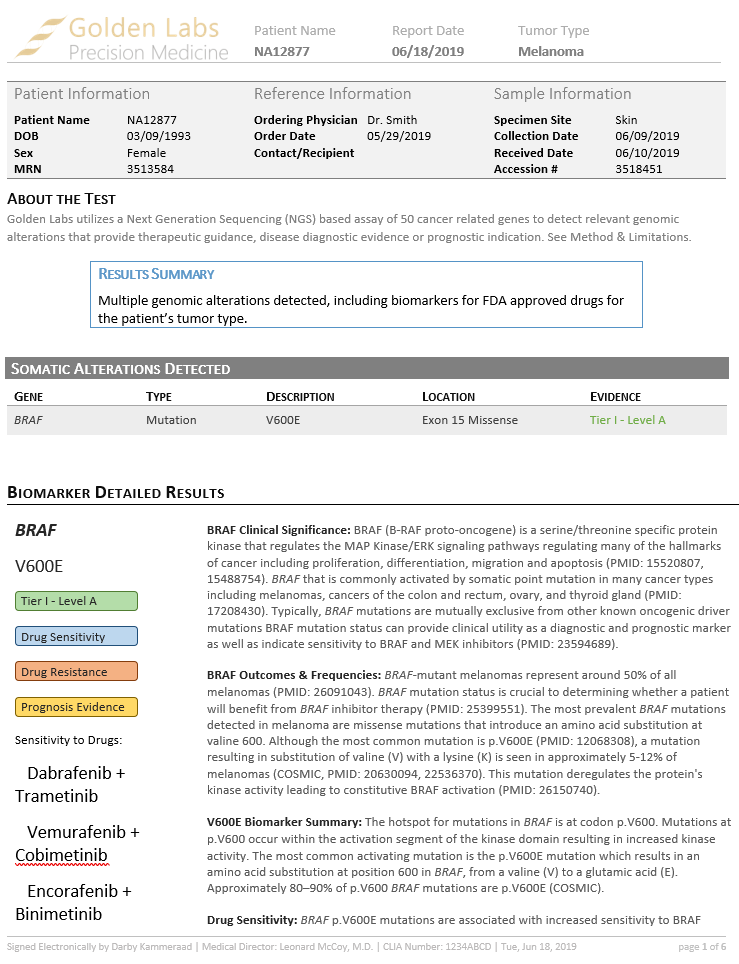
The AMP Guidelines also assist in classifying biomarkers by their clinical tier status according to the FDA Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer. This tiered system rates the clinical significance of different biomarkers, as well as the level of confidence in prognostic and diagnostic evidence relating to that biomarker.
Any Additional Features?
Two incredible new features of the AMP Guidelines are the Golden Helix CancerKB database provided to users and the new Word template report formatting feature.
Looking at oncogenic biomarkers first required the incorporation of different cancer-specific annotation databases like COSMIC, CIViC, Cancer Hotspots, PMKB, and ICGC, but Golden Helix also wanted to supply users with a solid repository of common biomarker interpretations that can be used to populate clinical reports. This is provided with the expert-curated CancerKB interpretation catalog that provides hand-written interpretations for the most common biomarkers and tumor types. This catalog will be regularly updated and will allow users to expand upon by checking the “Share with Curation Team” checkbox when saving their interpretations.
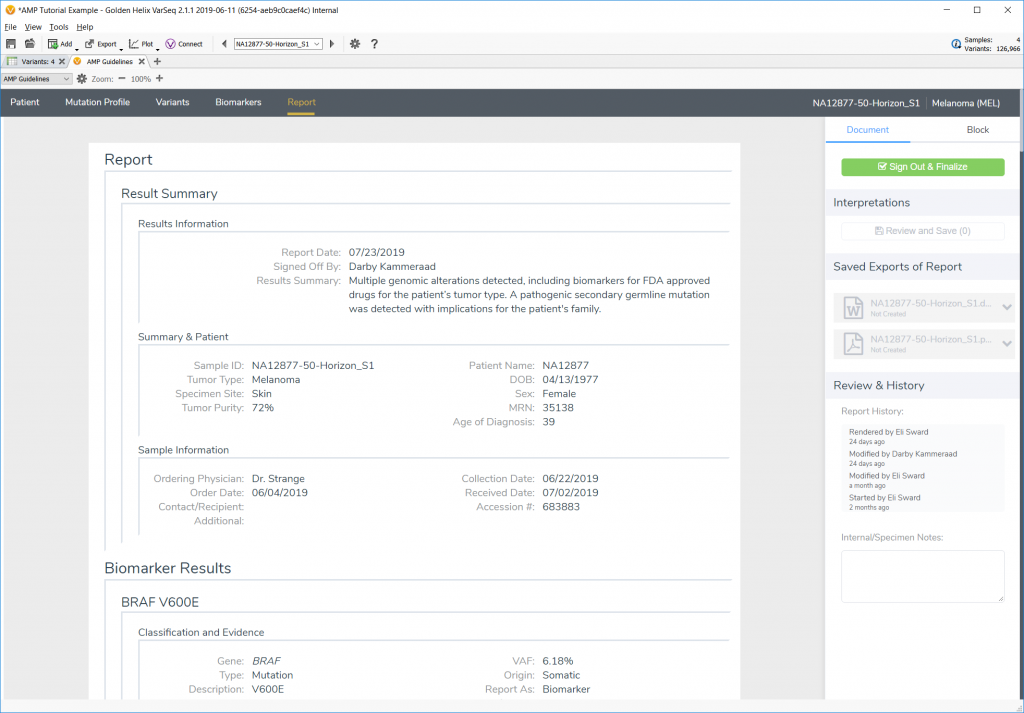
The second new feature is the ability to adjust and save report templates in Microsoft Word and then save complete reports as a Word document or as a PDF.
So, What Does the Tutorial Show?
The tutorial starts users off with a project that already has biomarkers loaded and ready to go, and then walks through four different example cases; two SNVs, one CNV, and one gene fusion.
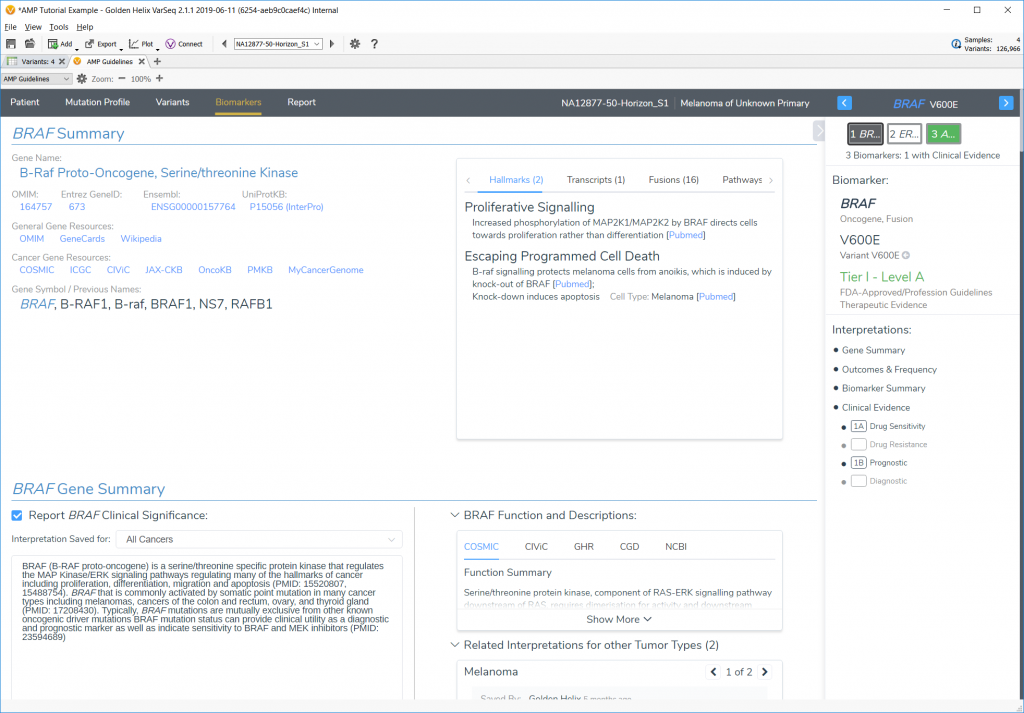
Each biomarker is explored, and interpretations added along the way to illustrate the capability of the AMP Guidelines and then arrive at the final report using a custom Word template.
We are excited to present this new tutorial for users to explore the features presented in VSClinical and incorporate the AMP Guidelines in their biomarker analyses!
If you are interested in getting a demo license and jumping into the AMP Guidelines with VSClinical, please request a demo let us know here and we will help you get started.