Gene Fusion Background
Gene fusions are hybrid genes that result from translocations, interstitial deletions, or chromosomal inversions that can lead to constitutive gene activation and result in increased or abnormal protein production. Increased or abnormal protein production subsequently can play an important role in tumorigenesis and thus identifying and evaluating this type of biomarker is important in the cancer workspace. For this blog, we will provide a case study for a gene fusion analysis and show how this biomarker can be evaluated with the VSClinical AMP workflow.
Case Scenario
A 50-year-old male presenting cough, sputum, and left chest pain was diagnosed with upper lobe cancer after a chest CT scan showed a lesion in the upper left lung. After resection and lymphadenectomy, the patient received chemotherapy, but due to poor tolerance, it was discontinued. The intraoperative specimen was examined by PCR and identified for the presence of the KIF5B/RET gene fusion. To determine if there are indications for treatment the KIF5B/RET fusion was imported into the VSClinical AMP Guideline workflow (1).
Importing the KIF5B/RET into VSClinical AMP
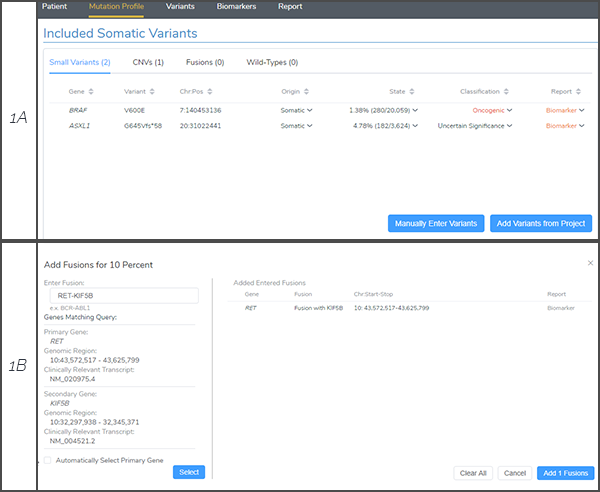
As outlined in the VSClinical AMP eBook, users have the ability to investigate and report on a variety of biomarker types including single nucleotide variants, indels, copy number variants, gene fusions and considerations for wild-type genes (Figure 1A). Since the KIF5B/RET was called by PCR methods, the fusion was added to the AMP-interface using the manual import option and RET was automatically detected as the primary gene (Figure 1B).
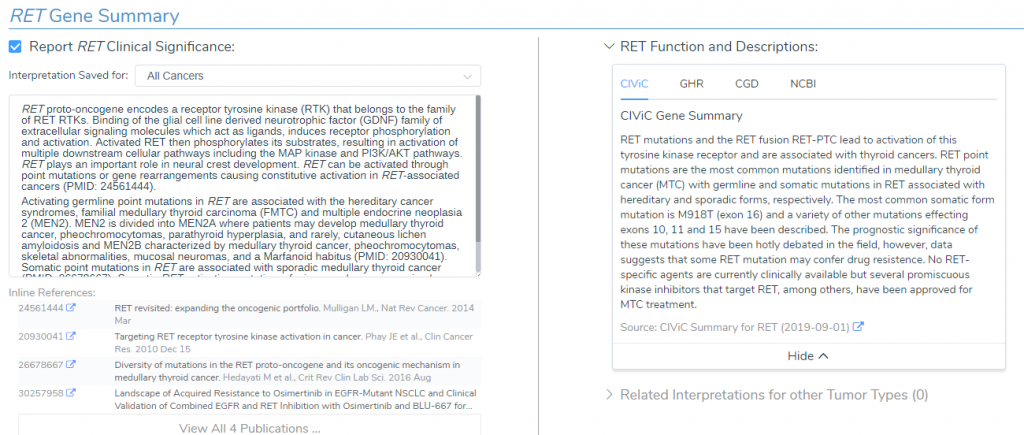
RET Gene Summary
The KIF5B-RET gene fusion is present in the CancerKB catalog, which is a carefully reviewed dataset containing assessments of biomarkers and genes built by an expert panel of curators. This catalog of aggregated evidence for the RET gene is pulled from CIViC, Genetic Home Reference, Clinical Genomic Database and NCBI (Figure 2). In summary, RET is a proto-oncogene that encodes a receptor tyrosine kinase (RTK) that binds to glial cell line derived neurotrophic factor (GDNF), which activates multiple intracellular pathways such as MAPK and PI3K/AKT. Additionally, somatic mutations in RET, such as fusions and overexpression, has been associated with cancer types including papillary thyroid carcinoma and lung carcinoma (Figure 2).
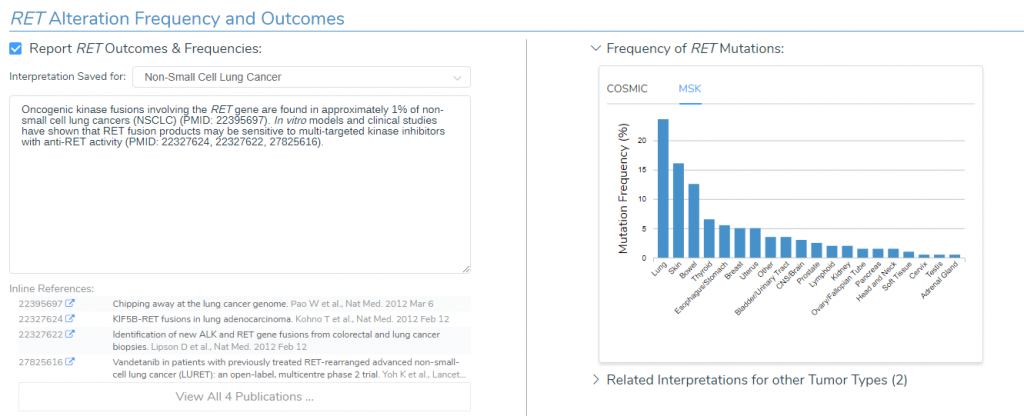
RET Alteration Frequency and Outcome
According to the Golden Helix CancerKB catalog, oncogenic kinase fusions involving the RET gene are found in roughly 1% of non-small cell lung cancers and in vitro models have shown that RET fusion products may be sensitive to kinase inhibitors with anti-RET activity. Within the Alteration and Frequency Outcome interface, we can also see that RET mutations predominate in the thyroid according to COSMIC and in the lung according to Memorial Sloan Kettering Cancer center (MSK) (Figure 3).

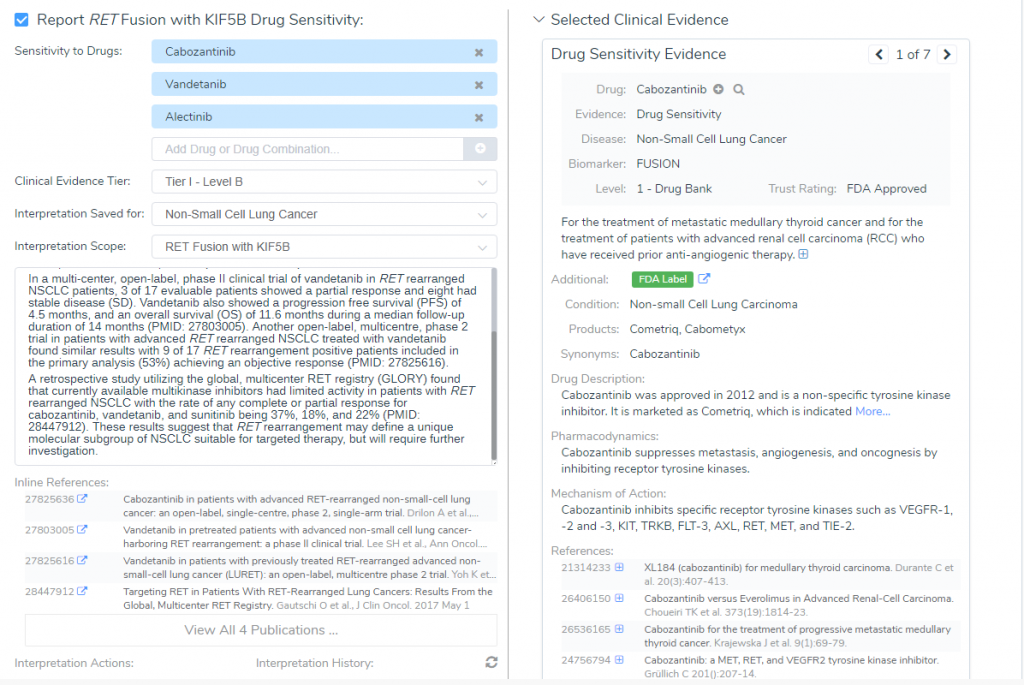
RET Fusion with KIF5B Clinical Evidence
In the “Clinical Evidence” section, GoldenHelix CancerKB provided two FDA Approved drugs for the RET-KIF5B fusion: Cabozantinib and Vandetanib. According to the descriptions from Drug Bank, Cabozantinib is used in the treatment of metastatic medullary thyroid cancer and for advanced renal cell carcinoma (Figure 4). Furthermore, a phase II trial of 26 patients with lung cancer harboring the RET rearrangements were treated with cabozantinib, which lead to a partial response in 28% of patients (3). On the other hand, Vandetanib is currently approved as an alternative to local therapies for both unresectable and disseminated disease. However, since Vandetanib can prolong the Q-T, it is contradicted for use in patients with serious cardiac complications. Together, the AMP feature provided two FDA approved drugs with well-powered studies with consensus from experts in the field, which brings the RET-KIF5B biomarker fusion to a Tier I- Level B classification.
Through the VSClinical AMP workflow, we were able to manually import a RET-KIF5B gene fusion that was identified using PCR methods and reach a Tier I- Level B interpretation with the FDA-approved drug, Cabozantinib. With the gene summary, frequency, outcome, biomarker and clinical evidence provided by Golden Helix CancerKB we can render a clinical report using this information (Figure 5).

With the VSClinical AMP feature we evaluated a RET-KIF5B gene fusion associated with non-small cell lung cancer and showed, through using the CancerKB catalog, how easy it was to classify this rearrangement as a Tier I – Level B mutation and render a clinical report. If you are interested in other capabilities such as filter and annotating, evaluating germline variants using ACMG guidelines, or calling copy number variants using your NGS data, please check out the hyperlinks. And as always, if you have any questions or would like to set up a meeting, please feel free to reach out to [email protected].
References
- Yucong Wang, et al. RET fusion in advanced non-small-cell lung cancer and response to cabozantinib. Medicine (2019); 98:3.
- Piotrowska Z, et al. Landscape of Acquired Resistance to Osimertinib in EGFR-Mutant NSCLC and Clinical Validation of Combined EGFR and RET Inhibition with Osimertinib and BLU-667 for Acquired Fusion RET. Cancer Discovery (2018); 12.
- Drilon A, et al. Cabozantinib in patients with advanced RET-rearranged non-small cell lung cancer: an open-label, single centre, phase 2, single-arm trial. Lancet Oncol (2016); 17(12):1653-1660.
- Mulligan LM, et al. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer (2014); 12(3):173-86.
- Kohno T, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med (2012); 18(3):375-7.