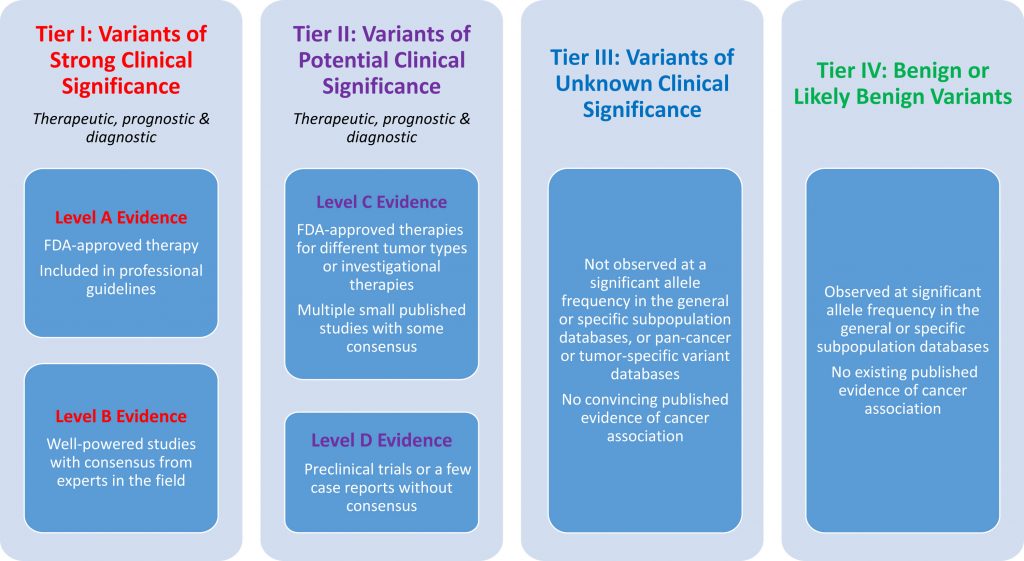
The AMP guidelines workflow in VSClinical provides a user-friendly tool for the interpretation of somatic biomarkers across the entire spectrum of genomic variation. One of the most useful features of this workflow is its ability to streamline the evaluation of clinical evidence for a somatic biomarker using the AMP Tier evidence levels. The AMP Guidelines classify a biomarker into one of four tiers based on the level of available clinical evidence.
For many cancer mutations, this classification process is largely automated by pulling data from our Cancer Knowledge Base (CancerKB), which provides comprehensive interpretations for many common cancer genes and biomarkers for specific tumor types. However, for lesser-known variants, more work is needed to assess the available clinical evidence.
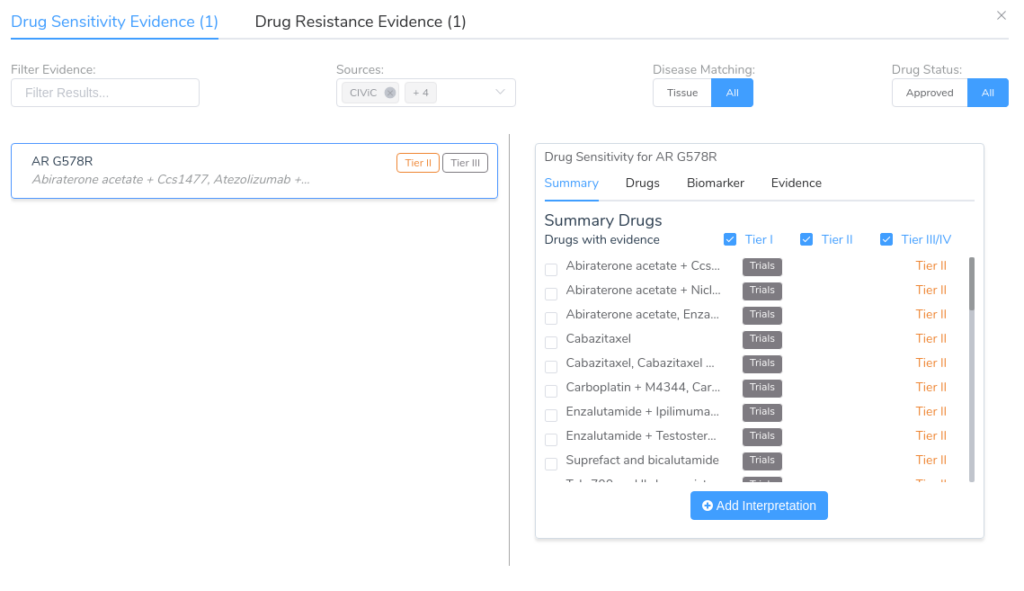
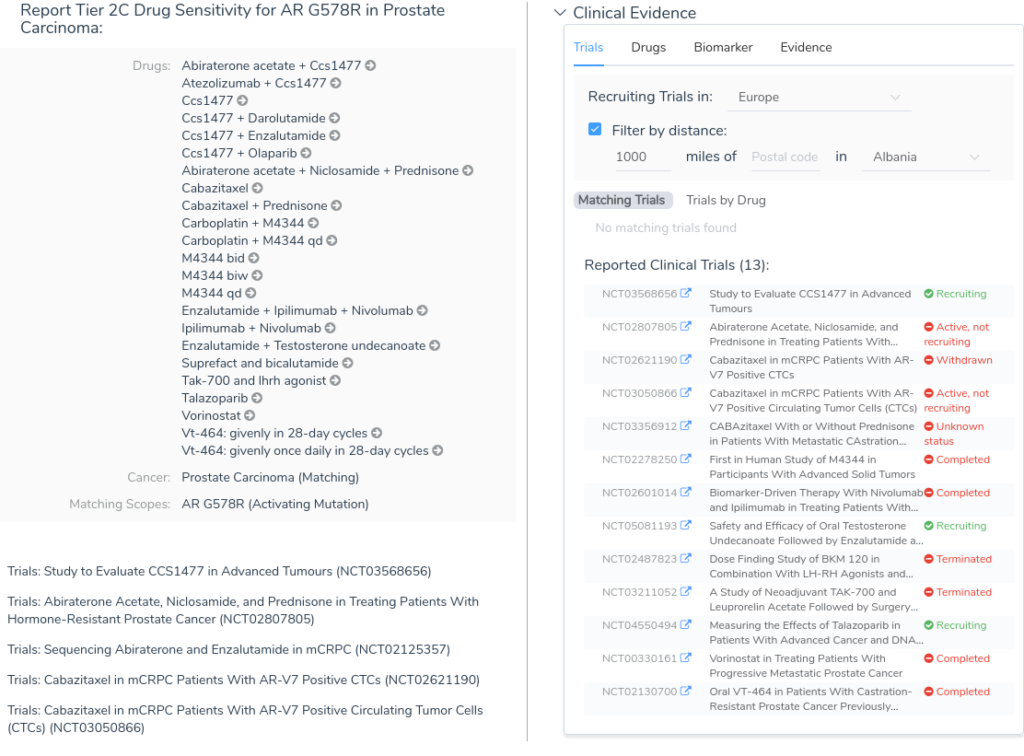
The Clinical Evidence tab of the AMP workflow allows users to search for clinical evidence across a number of different data sources, including CIViC, Drug Bank, Clinical Trials, and CancerKB. In the example below, we see the drug sensitivity search results for the variant AR G578R in a prostate carcinoma sample.
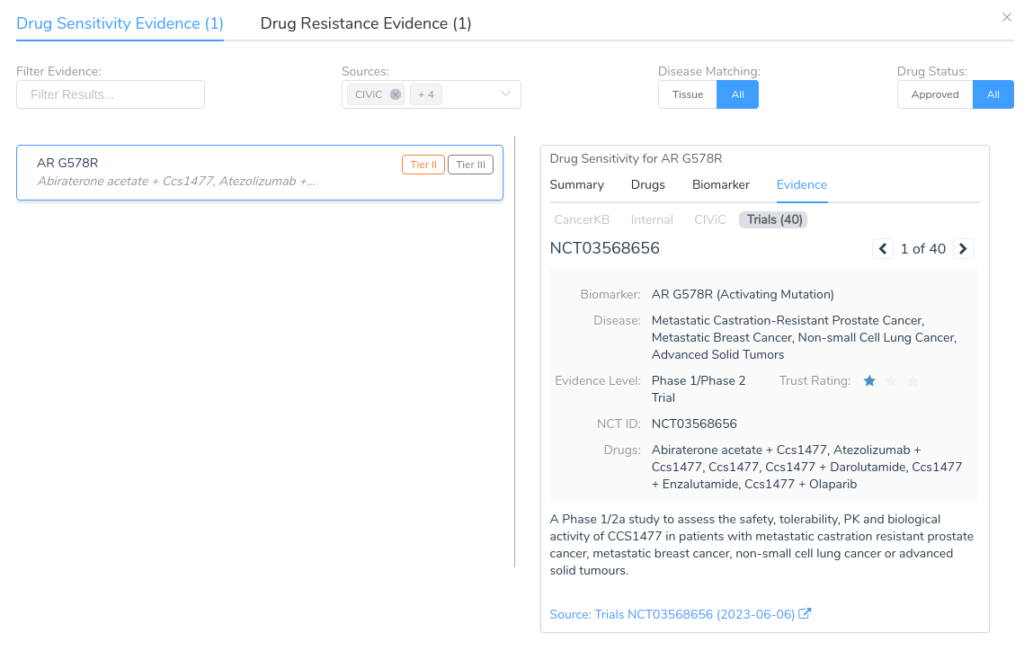
While this variant lacks any directly associated evidence in CancerKB, there is a wealth of clinical evidence coming from a large number of clinical trials. Most users will want to examine this evidence critically, investigating each individual clinical trial before adding the evidence to the evaluation. The details for each piece of clinical evidence can be explored using the Evidence tab, as shown below.
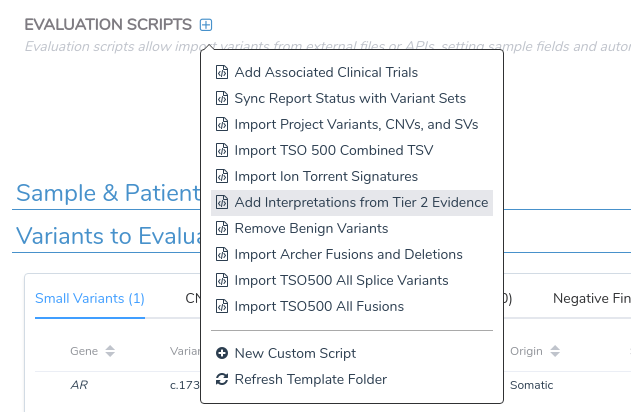
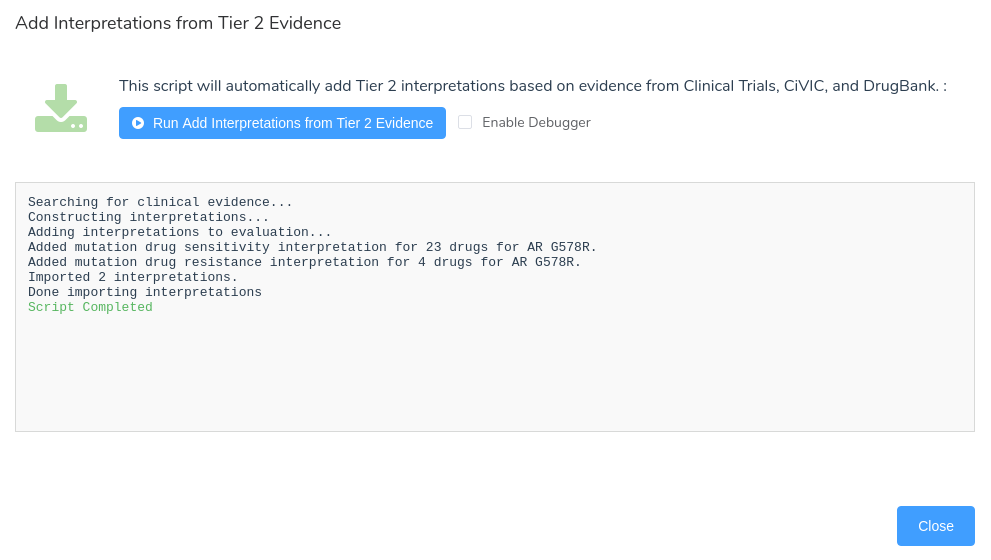
However, some users may wish to automatically incorporate this evidence into the evaluation without manually selecting each individual search result. To support this functionality, we have added a new evaluation script to VSClinical that automatically adds this type of secondary evidence to the current evaluation. This script can be added to a project through the Evaluation Scripts menu in the Evaluation tab.
Once added, we can click the Run button to open the script dialog, where we can execute the script and import all available Tier 2 evidence.
After running the script, the dialog will display details describing the script’s progress and, when completed, will list all added interpretations.
For this interpretation, we can see that the script added a drug sensitivity interpretation containing all 23 drugs listed in clinical trials associated with the AR G578R biomarker, along with a drug resistance interpretation for 4 drugs. Returning to the Clinical Evidence tab, we can examine the details for the newly added drug sensitivity interpretation. The text of the interpretation includes a reference to each of the selected clinical trials, and all of the associated trials have been added to the evaluation.
By default, this script only adds clinical evidence estimated to have a Tier 2 classification, but users possessing rudimentary JavaScript experience can modify the script to include Tier 3 evidence as well. As with all of our evaluation scripts, the functionality can be extended to suit the needs of each individual lab.
In this blog post, we introduced a new evaluation script that enables users to automatically incorporate secondary evidence into their evaluations. This customizable script automatically pulls in all Tier 2 clinical evidence from DrugBank, CIViC, and Clinical Trials, streamlining the process of incorporating this evidence into your evaluation. If you have any questions about evaluation scripts in VSClinical or about the AMP Guidelines in general, please don’t hesitate to contact us.