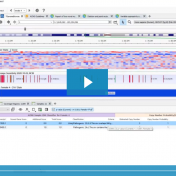
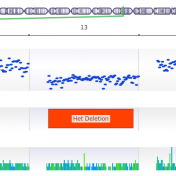
In our previous webcast, Evaluating CNVs with VSClinical’s New ACMG Guidelines, we focused on a CNV deletion (12:27715515-29628122×1) in which the patient had a known disorder called Brachydactyly type E. The CNV was isolated using our VS-CNV caller and applied to the ACMG CNV guidelines using the intuitive steps of VSClinical. If you missed the webcast, you can watch the… Read more »
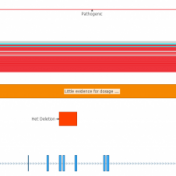
In the webcast, Evaluation of Copy Number Variants with VSClinical’s New ACMG Guideline Workflow, we discussed how VSClinical implements Section 4 of the ACMG guidelines. Specifically, we focused on integrating literature and publications to assess the pathogenicity of a CNV event when there was a lack of dosage sensitivity information. One of the primary pieces of evidence for evaluating genes… Read more »
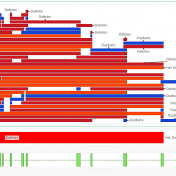
Golden Helix is excited to release an upcoming VSClinical feature that allows users to analyze next-generation sequencing (NGS) CNV event reporting with ACMG guidelines. This feature will be the first in the NGS workspace to allow this capability and if you are curious about the functionalities you can get a sneak peek by looking at some of our most recent… Read more »
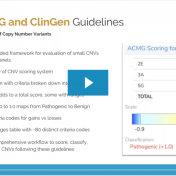
In a recent webcast, users were exposed to some new features upcoming in the next release of VarSeq. In this update, we will use an example de Novo CNV in a cardiomyopathy panel in VarSeq. There is a long list of new tools and polishes to the software but one major upgrade is the inclusion of the ACMG and ClinGen… Read more »
Our previous webcast from VP Gabe Rudy in September exposed us to some fundamentals of this years’ updated Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). This recent webcast was dedicated to breaking down these new guidelines… Read more »
In our recent webcast announcing the upcoming release of VarSeq VSClinical and the implementation of the ACMG guidelines for NGS CNVs, we had a number of live questions we didn’t get a chance to cover at the end of the presentation. I will follow up on those questions in this blog post. But first, if you didn’t get a chance to join us for… Read more »
Our software solutions and partners have brought dramatic improvements to the secondary and tertiary analysis stages of variant evaluation. Regarding secondary analysis, we’ve discussed increased efficiencies in speed and overall accuracy in the variant calling process with Sentieon. On the tertiary side, we have explored numerous workflows in VarSeq highlighting filtration to clinically relevant variants, as well as the automated… Read more »
We have had many customers come to us over the years with a simple problem: they have BAM files for whole exome or gene panel data and would like to call CNVs using VarSeq’s powerful CNV calling capabilities, but they don’t have a bed file defining the target regions for their samples. To address this problem, we have developed a… Read more »
Copy Number Variation (CNV) is a type of structural variation in which sections of the genome are duplicated or deleted. Although CNV events are rare in the human population, constituting approximately 10% of the human genome, they are also associated with being causal mutations for disease phenotypes. Because of this, it is important for clinical and research settings to identify… Read more »
We are happy to announce that our latest version of SVS includes the ability to call CNVs on low read depth Whole Genome Sequencing (WGS) data. Designed for calling large cytogenetic events, this algorithm can detect chromosomal aneuploidy events and other large events spanning one or more bands of a chromosome from genomes with average coverage as low as 0.05x…. Read more »
We love when our viewers send questions in during the webcast but unfortunately we can’t answer all of them during the time allotted! If you asked a question see below for answers, or if after viewing, you have any questions that weren’t asked, please feel free to send those over to support@goldenhelix.com. Does this work for FFPE derived DNA or ctDNA?… Read more »
We recently hosted a webcast covering the value and application of VSWarehouse through VarSeq. Not only is VSWarehouse a solution for storing your NGS data in a central repository, but it also provides a means to enhance the tertiary analysis done in VarSeq. VSWarehouse will store all your sample/variant data but also stores your catalogs of pathogenic variants, clinical reports, and has the capability of filtering/querying on all your stored data quickly. In addition,… Read more »
In my recent webcast, I demonstrated how VS-CNV users can detect high-quality copy number variant events. If you didn’t have a chance to join, you can view the recording below! This webcast generated a lot of great questions! If you have any other questions about the content covered in this webcast that is not answered below, please feel free to… Read more »
With this two-part blog series, users should now be able to perform CNV analysis using their data, set up basic quality filter standards to isolate high-quality events and utilize annotations to hone in on publicly known events as well as in-house recorded CNVs from previous projects.
2017 was a busy year regarding the development of our CNV tools. Since the release of the CNV caller, we have produced quite a bit of content tailored to assist our users with getting started. Here are some links: Robarts Research Institute CNV analysis on patients with familial hypercholesterolemia CNV annotations Common CNV questions CNV calling with shallow whole genome… Read more »
First-place Abstract Competition Winner, Michael Iacocca, shared his research with the Golden Helix Community during our February webcast ‘Using NGS to detect CNVs in familial hypercholesterolemia‘. In this webcast, he gave a great explanation on how our CNV caller aided his team in their research. If you were unable to join us for the event, you can find a recording… Read more »
One of our main focuses in 2017 was VS-CNV which allows clinicians to directly call CNVs in target regions quicker, easier and more affordably than CMA or MLPA testing. Our clients at Robarts Research Institute shared their recent publication with me which confirms that our time and dedication to our CNV capabilities was well worth it. I am delighted to… Read more »
The Golden Helix SNP & Variation Suite (SVS) platform is a powerful and versatile set of tools and algorithms for performing genomic research. That research spans from data originating on genotype micro-arrays to next-generation sequencing. While the majority of SVS users start with genotype data on their samples, any genomic information across a cohort can be used in our various… Read more »
With the recent upgrade to VarSeq 1.4.7, users gain access to some new great features. Among the additions are new CNV annotations (Figure 1). In this final chapter of the annotation blog series, we are going to provide descriptions of the new CNV annotations and how they can be used. The types of CNV annotations vary and include frequency, clinical… Read more »
VarSeq Updated with CNV Annotations, CNV PhoRank, and Region Assessment Catalogs This year we have released multiple advances to support CNV analysis in VarSeq, expanding our target region based VS-CNV caller to handle exomes and low-depth genomes as well as additional supporting algorithms like calling Loss of Heterozygosity regions. To top this off, VarSeq 1.4.7 has been shipped with many… Read more »