Awarded one of the top biotech companies Insight Success recently published its annual Pharma and Life Special Edition announcing the 10 most innovative solution providers of 2018. We are incredibly honored to have received this award and being recognized amongst the top biotech companies! You can access the publication featuring my interview with the editor here: http://www.insightssuccess.com/golden-helix-helping-researchers-clinicians-understand-role-cnvs-human-health-disease/. I have outlined some… Read more »
2017 was an incredibly prosperous year for Golden Helix; we released a handful of new features, announced new partnerships and completed our end-to-end architecture for clinical testing labs. Our webcast series has become a very popular way for our community to stay up-to-date with our new capabilities and best practices in genetic analysis using our software. We had three webcast… Read more »
The Golden Helix SNP & Variation Suite (SVS) platform is a powerful and versatile set of tools and algorithms for performing genomic research. That research spans from data originating on genotype micro-arrays to next-generation sequencing. While the majority of SVS users start with genotype data on their samples, any genomic information across a cohort can be used in our various… Read more »
First of all, I wish you a prosperous 2018 along with happiness and health for you and your loved ones. This next year comes with lots of anticipation. We at Golden Helix are looking forward to another year of growth and innovation. Over the last few years, we were able to build a large following of clients in the clinical space…. Read more »
As VarSeq is quickly becoming the go-to variant analysis software for tertiary analysis, we want to give our readers the opportunity to examine completed projects from start to finish. As an addition to the currently available demonstration projects, we are pleased to provide users with a Single Exome Analysis example project. To access this project simply click here to download… Read more »
Clinical Assessment Tracks Golden Helix provides a large catalog of annotation sources for our research and clinical clientele. Making these public data repositories available to all our users is no easy task. As Cody Sarrazin mentioned in his blog post, annotation curation is a complex data science pipeline. This process aggregates data from many disparate sources and normalizes it into… Read more »
Next-Gen Sequencing promised to be the ultimate paradigm when it comes to genetic research and clinical testing since it contains the complete genetic information. When it comes to the current reality in testing labs, there are still a number of additional testing paradigms used in an analysis, specifically, copy number variations. Among these, labs still widely use Chromosomal Microarrays and… Read more »
An Example of an Integrated Clinical Workflow for CNVs and SNVs In this blog series, I discuss the architecture of a state of the art secondary pipeline that is able to detect single nucleotide variations (SNVs) and copy number variations (CNVs) in one test leveraging next-gen sequencing. In Part I, we reviewed genetic variation in humans and looked at the key… Read more »
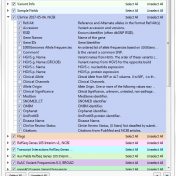
Examples of CNV Calling What do CNV calls actually look like? What are some of the key metrics to determine an event? Part IV of the Secondary Analysis 2.0 blog series will answer these questions by walking through some examples of how our CNV caller, VS-CNV, identifies CNVs. Golden Helix integrates multiple metrics to determine if a CNV event is… Read more »
Detection of CNVs in NGS Data Our Secondary Analysis 2.0 blog series continues with Part III: Detection of CNVs in NGS Data. We will give you an overview of some design principles of a CNV analytics framework for next-gen sequencing data. There are a number of different approaches to CNV detection. The published algorithms share common strategies to solve the… Read more »
In this blog series, I will discuss the architecture of a state of the art secondary pipeline that is able to detect single nucleotide variations (SNV) and copy number variations (CNV) in one test leveraging next-gen sequencing. In Part I, we reviewed genetic variation in humans in general and looked at the key components of a systems architecture supporting this… Read more »
Human genetic variation makes us unique. On average, humans are to 99.9% similar to each other. Understanding in detail what the nature of the difference in our genetic make-up is all about allows us to assess health risks, and eventually enables Precision Medicine as we determine treatment choices. Furthermore, it enables scientists to better understand ancient human migrations. It gives… Read more »
CIO Review recently published its annual Biotech Technology Special Edition announcing the 20 most promising biotech technology solution providers. It is a great honor that Golden Helix has been named among the top 20 for the second year in a row! Please find my interview with the editor here: Spearheading Innovation in the Biotech Industry We are very thankful for the hundreds of organizations and… Read more »
Genome-wide association study (GWAS) technology has been a primary method for identifying the genes responsible for diseases and other traits for the past ten years. GWAS continues to be highly relevant as a scientific method. Over 2000 human GWAS reports now appear in scientific journals. In fact, we see its adoption increasing beyond the human-centric research into the world of… Read more »
The year 2017 is starting fast and furious for us here at Golden Helix. We just announced a new imputation capability for our SVS product. At the same time, members of our team are on the way to PAG in San Diego to network with our clients in the Plant and Animal community. We have a terrific plan in place… Read more »
Golden Helix closed out 2016 with a great honor; in December, The Silicon Review Magazine released a special edition naming Golden Helix as one of the fastest-growing technology companies for 2016. You can read the interview with our CEO, Andreas Scherer, Ph.D, here: Delivering industry-leading analytic software and services. We continue to believe that our customers are paramount in this honor, as… Read more »
Today, we launched our eCommerce Store. With this capability we respond to our customers requesting a simplified way to conduct business with us. Here is the background on this latest development. As we continue to grow in the genomics space, the needs of our clients fall really into two categories. On one hand, there are clients who want convenient access to our… Read more »
Last week, I attended the Advances in Medical Genetics conference in Riyadh, Saudi Arabia. I was asked to present on “Big Data in DNA Analytics”. The event was hosted by Prof. Dr. Majid Alfadhel of the King Saud Bin Abdulaziz University for Health Sciences in collaboration with the Postgraduate Training Center. The event was held to discuss the pros and cons surrounding the… Read more »
Wednesday, March 2nd 12:00 pm EST Clinical labs must have the ability to go from a collection of samples and associated variants to a professional report documenting a short list of clinically relevant variants. Cancer Gene Panels are a common clinical application for genetic tests. In this webcast we will show how VarSeq and VSReports can be used to go… Read more »
Like most, we at Golden Helix love this time of year! It is a time to spend with family and friends, creating memories and reflecting on the past year. From all of us here, to you and yours, we wish you a very happy holiday and a prosperous 2016!