Our tutorials are a popular resource for prospective users. We have added a tutorial to help introduce customers to the ease and utility of the ACMG Guidelines incorporated in VSClinical. The ACMG Guidelines are a collection of 33 criteria that are used to determine a variant’s level of pathogenicity. VSClinical and VarSeq make it easy for users to sort and… Read more »
To recap what we have covered in this blog series thus far, Sentieon allows users to call somatic variants against a matched normal sample and a tumor-only analysis. Part I of this series covered the variant calling workflow for tumors with matching normal samples. However, a common situation would be to call variants for a tumor sample without the normal…. Read more »
SVS is a project-oriented program that manages and analyzes genomic datasets. This webcast statistically and visually explores the relationships among genetic variants within a cattle dataset. Even further, this webcast evaluates genotypes with corresponding phenotypes to assess how well a model can predict a phenotype of interest. Starting with genotypic data from the microarray and the recorded phenotypic data for… Read more »
Our team has returned from the annual meeting of the Association of Molecular Pathology (AMP 2019), and, as always, I am grateful for all the wonderful experiences we are bringing back with us. The plenary sessions and talks were bountiful, and we were very impressed with the well-organized exhibition. Hats off to everyone involved in planning this great event! Innovation… Read more »
We have recently added a tutorial to help introduce customers to the ease and utility of the AMP Guidelines incorporated in VarSeq’s VSClinical package. The AMP Guidelines allow users to sort through available clinical evidence in a streamlined fashion to arrive at final classification and interpretation and then transfer that information into a clinical report. And the AMP Guidelines also… Read more »
As I mentioned in the first part of this series, Sentieon allows users to call somatic variants against a matched normal sample and a tumor-only analysis. Utilization of a Tumor-Normal Workflow In addition to the fundamental process of alignment and variant calling, there are a few more steps that will improve the quality of your secondary analysis. Figure 1 (below)… Read more »
With October being recognized as Breast Cancer Awareness Month, the team at Golden Helix thought it would be appropriate to pay tribute to some of the publications that over the years, have contributed to the many discoveries made in breast cancer diagnosis, prediction, underlying causes, and case management. We are very proud to be a part of cancer research and… Read more »
Golden Helix works to keep incorporating and updating great somatic annotation catalogs for our VSClinical users. We currently have the updated version of one of the largest cancer databases from the International Cancer Genome Consortium, or ICGC. Version 28 has been improved by integrating ClinVar and CIViC clinical annotations, and as always, increasing the number of mutations listed. The current… Read more »
VarSeq 2.2.0 was released today and this a stable release full of upgrades and polishes. Some of the newer features include the ability to store and include AMP Cancer assessment catalogs on VSWarehouse, quicker accessibility to common annotations plotted in GenomeBrowse, and the addition of all of our standard templates for the GRCh38 genome assembly. Many of the polishes were… Read more »
As our team returns from another successful ASHG conference, I would like to reflect on the great memories, connections, and future plans that were made at this meeting. First, I will start by thanking everyone involved with the superb planning and execution of the conference. We are thankful to have this opportunity to connect with our customers, partners, and friends… Read more »
Our team is getting ready to head to Houston, Texas for the American Society of Human Genetics Annual Meeting (ASHG) 2019. We are looking forward to spending the week discussing genetics with new and familiar faces! If you are planning on attending, please stop by our booth and say hello! You will find the Golden Helix team in booth 601…. Read more »
The common approaches to detecting copy number variants (CNVs) are chromosomal microarray and MLPA. However, both options increase analysis time, per sample costs, and are limited to the size of CNV events that can be detected. VarSeq’s CNV caller, on the other hand, allows users to detect CNVs from the coverage profile stored in the BAM file, which allows you… Read more »
Sentieon has become well known for the dramatic improvements it supplies in speed and accuracy for secondary analysis. Luckily, these improvements are not only available for DNAseq germline based variants but also for TNSeq somatic investigation. Sentieon has proven the capability by leading in awards such as the ICGC-TCGA DREAM Challenge for SNVs, Indel, and structural variants. This value not… Read more »
Before examining the clinical evidence associated with a specific mutation, a clinician must establish that the variant is likely to be a driver mutation which generates functional changes that enhance tumor cell proliferation. Our recent blog series “Following the AMP Guidelines with VSClinical” briefly mentioned how the oncogenicity scoring system in VSClinical could be used to automate and assist the… Read more »
From diagnosing, treating and preventing diseases in humans and animals, to preserving our global food supply, Golden Helix products are being used all over the world! In September, we were cited in many publications spanning a diverse range of species and topics. Below are brief recaps of a few of the studies where SNP & Variation Suite (SVS) and VarSeq… Read more »
Customers are always asking for ways to improve their experience with Sentieon, our partner’s secondary analysis tools that process genomics data with high computing efficiency, fast turnaround time, exceptional accuracy and 100% consistency. We have a few tips to get the most out of Sentieon. In this article I will be going over: Before you set up your environment you… Read more »
Gene Fusion Background Gene fusions are hybrid genes that result from translocations, interstitial deletions, or chromosomal inversions that can lead to constitutive gene activation and result in increased or abnormal protein production. Increased or abnormal protein production subsequently can play an important role in tumorigenesis and thus identifying and evaluating this type of biomarker is important in the cancer workspace…. Read more »
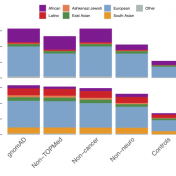
The Broad Institute team led by Dan MacArthur announced the release of gnomAD version 2.1 at last year’s ASHG conference. This new version boasted data from 125,748 exomes and 15,708 genomes, but the greater updates were the improved QC refinement and more discrete sub-population break downs. Although the majority of samples were counted in the previous 2.0.2 release, the additional… Read more »
We have covered a lot of ground in this Automating & Standardizing Your Workflows blog series. First, we saw how to perform secondary analysis with Sentieon to generate the necessary VCF and BAM files for tertiary analysis in Part I. The implementation of VSPipeline allowed for rapid import and project generation for a predefined cancer gene panel project template in… Read more »
VSClinical users can interpret and report genomic mutations in cancer following the AMP guidelines which we’re demonstrating in this “Following the AMP Guidelines with VSClinical” blog series. Part I introduced the hands-on analysis steps involved in creating a high-quality clinical report for targeted Next-Generation Sequencing (NGS) assays. We reviewed sample and variant quality, including the depth of coverage over the target regions by the sequencing performed for each sample. Now, we are ready to… Read more »