As the world is consumed by the ongoing pandemic, it is easy to forget that there are investigators all around the globe that continue to make important discoveries in human medicine. Below are a few examples that remind us there are those that persevere in their chosen fields of study despite the trying times. At Golden Helix, we continue to… Read more »
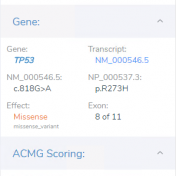
Did you know you can control your preferred transcript settings for clinical interpretation in VSClinical? Your lab is analyzing the DNA of a tissue sample from a patient with small cell lung cancer. The lab technician has imported the patient data into VSClinical to detect clinically relevant variants and evaluate and score these according to the AMP Guidelines, as well… Read more »
Golden Helix has secured its reputation as a global leader in Next-Generation Sequencing (NGS) solutions for over two decades. Today, we are proud to announce that we have been included in the esteemed Inc. 5000 List of rapidly growing American companies. Out of the 6 million businesses in the United States, Golden Helix has been honored with a spot in… Read more »
Golden Helix software provides huge analytic gain in handling large-scale genomic data. For example, a number of VarSeq users run cohort projects of whole genome level data processing hundreds of millions of variants at a time. However, many of our users are running gene panel level data for custom panels related to cancer (both hereditary and somatic), autism, cardiac, and… Read more »
In this month’s Customer Publications blog post, our VarSeq software is taking center stage! From whole exome sequencing to copy number variant calling, VarSeq can be used for a range of scientific investigations. Although this blog features several examples of cancer investigations in human patients, it’s interesting to see how this platform can be utilized in a variety of investigational… Read more »
It is common knowledge that variants can be germline or somatic depending on whether the variant was inherited or acquired after birth. A well-known example is cancer-causing mutations in the BRCA genes, wherein the mutation may or may not have been inherited. Understanding the origin of the cancer-causing mutation is important when assessing potential treatment options as well as identifying… Read more »
Introduction: Malignant Rhabdoid tumors (MRT) are among the most aggressive and lethal forms of infant and child cancer (1). These tumors are characterized by an unusual combination of mixed cellular elements similar to but not typical of teratomas and can originate at any anatomic location. When MRTs are present in the brain, they are called atypical teratoid/rhabdoid tumors (AT/RT), which… Read more »
Golden Helix has been incredibly fortunate to have been featured in a variety of publications over these last six months. Topics span from the history and future of our company to several new use cases for our solutions that extend into the infectious disease space, recently coming to fruition with the COVID-19 pandemic. We are so grateful to have received… Read more »
As I prepared to write the Customer Publication blog for June 2020, I was excited by the number of recently published papers that stood as examples of how both VarSeq and SVS software are employed to advance diagnostics and treatments in human medicine. We often think of SVS as the go-to platform for Agrigenomics, however both of our platforms have… Read more »
It is an honor to be featured in the Clinical OMICs May/June 2020 issue in a Q&A with the Editor discussing the past, present, and future of Golden Helix. In this article, I detail: Clinical OMICs Article: What has been Golden Helix’s most significant success or contribution to the industry over the past five years? Golden Helix started in 1998 with a… Read more »
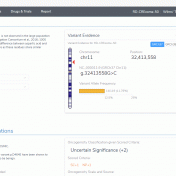
Abstract Before assessing the clinical significance of a somatic mutation, one must determine if the mutation is likely to be a driver mutation (i.e. a mutation that provides a selective growth advantage, thereby promoting cancer development). To aid clinicians in this process, VSClinical provides an oncogenicity scoring system, which uses a variety of metrics to classify a given somatic mutation… Read more »
Writing the blog post to summarize and highlight our customer’s publications is undoubtedly one of my favorite things to do! The wide variety of topics is always surprising and inspiring, and I am humbled by the efforts of dedicated scientists who are helping to protect and enrich our lives in so many ways. Our SNP & Variation Suite (SVS) software… Read more »
Today, we’re thrilled to announce that Healthcare Tech Outlook has named Golden Helix among the Top 10 Genetic Diagnostics Companies of 2020. This recognition places us alongside a remarkable group of companies that are leading innovation in genomics, diagnostics, and precision medicine. We are honored to receive this distinction and grateful for the opportunity to share our story in the… Read more »
Scientific investigations and genetic discoveries continue to happen in several diverse areas even while our world is currently consumed by the greatest health challenge of our era. Below are a few examples from April 2020, showcasing some of the great work being done in the fields of human medicine, ecology, and breed conservation of animal species. As always, we are… Read more »
VarSeq 2.2.1 was released on April 1st and features an upgraded gene annotation capability with new RefSeq genes tracks and an AMP workflow addition: the Drugs and Trials tab. The new RefSeq human genome genes tracks contain updated gene names and the recognition of any MANE (Matched Annotation from NCBI and EMBL-EBI) identified transcripts. VarSeq has been updated to be… Read more »
Our customers are making great contributions to human research and precision medicine every month! In February 2020, VarSeq’s Clinical Suite dominated the published works citing Golden Helix. It is an honor for us to be at the forefront of so many important genetic investigations and we appreciate the efforts of those who work for the greater good. Read further to… Read more »
Golden Helix is in a unique position to provide a secure on-premise analysis solution. This capability is based on two enablers. First, we build our software solutions from scratch and from the ground-up with the assumption that it should run on any operating system and potentially behind firewalls or even without internet access. Second, we provide these solutions on a licensing model based on training and supporting users, not… Read more »
We are incredibly grateful to be recognized as one of the Top 60 Genetics Blogs on the Web by Feedspot. Our team is dedicated to educating our readers on how our solutions can help enable precision medicine, and we are so honored to have received this recognition. On our blog, you will discover posts touching on important topics, like cyber security strategies,… Read more »
As many of our users know, GRCh38 VarSeq project templates come preloaded with the software and are designed to give users a baseline workflow to streamline their NGS analysis. These templates are tailored for various applications including tumor-normal, trios, cancer and hereditary gene panels, and ACMG Guidelines workflows. The templates contain application-specific annotation sources and algorithms that will automatically load… Read more »
Happy New Year! Certainly, I hope you had a relaxing time over the holidays with family and friends as well as a great start into the new year. 2019 was a real landmark year for Golden Helix. Please let me mention a few highlights: Now, for 2020 we will plan to continue to build on this success. Here are some… Read more »