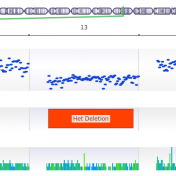
We have had many customers come to us over the years with a simple problem: they have BAM files for whole exome or gene panel data and would like to call CNVs using VarSeq’s powerful CNV calling capabilities, but they don’t have a bed file defining the target regions for their samples. To address this problem, we have developed a… Read more »
In VarSeq 2.2.1, you can enable auto-updating for template annotation sources, ensuring they always reflect the latest available version. Previously, VarSeq templates were frozen in time. Now, each new project created from a template would use the same source that was used when the template was created. When you save a template, you can have the sources automatically update to… Read more »
Our Support Team curates a variety of tutorials to help orient new users to the capabilities of VarSeq. We are happy to announce the team’s new release of the trio tutorial that places emphasis on using the ACMG guidelines. This tutorial gives insight into the proper setup of pedigree structure as well as detailed descriptions of the filter containers and… Read more »
Generating a clinical report is the final step of most NGS pipelines and is important as it relays results and information to legacy systems, physicians and ultimately the patient. As reporting is a valuable process, Golden Helix offers reporting capabilities according to the ACMG and AMP guidelines but also as a standalone feature in VSReports. VSReports is a platform that… Read more »
At Golden Helix, we want our new users to hit the ground running with VarSeq and not spend oodles of time getting started building and automating their workflows. To achieve this goal, our team has generated blogs, webcasts, and tutorials that explain and demonstrate workflows that are possible with VarSeq. Each VarSeq tutorial offers step by step instruction in which… Read more »
Since the initial release of the copy number variant algorithms in VarSeq, our team has created a variety of content to help users get started with building their copy number variant projects. In our webcast library, you can find a few of our recent webcasts in 2019 covering CNV workflows and validation: Or, if you prefer to take a more… Read more »
As many of our users know, GRCh38 VarSeq project templates come preloaded with the software and are designed to give users a baseline workflow to streamline their NGS analysis. These templates are tailored for various applications including tumor-normal, trios, cancer and hereditary gene panels, and ACMG Guidelines workflows. The templates contain application-specific annotation sources and algorithms that will automatically load… Read more »
Our tutorials are a popular resource for prospective users. We have added a tutorial to help introduce customers to the ease and utility of the ACMG Guidelines incorporated in VSClinical. The ACMG Guidelines are a collection of 33 criteria that are used to determine a variant’s level of pathogenicity. VSClinical and VarSeq make it easy for users to sort and… Read more »
We have recently added a tutorial to help introduce customers to the ease and utility of the AMP Guidelines incorporated in VarSeq’s VSClinical package. The AMP Guidelines allow users to sort through available clinical evidence in a streamlined fashion to arrive at final classification and interpretation and then transfer that information into a clinical report. And the AMP Guidelines also… Read more »
The common approaches to detecting copy number variants (CNVs) are chromosomal microarray and MLPA. However, both options increase analysis time, per sample costs, and are limited to the size of CNV events that can be detected. VarSeq’s CNV caller, on the other hand, allows users to detect CNVs from the coverage profile stored in the BAM file, which allows you… Read more »
We have covered a lot of ground in this Automating & Standardizing Your Workflows blog series. First, we saw how to perform secondary analysis with Sentieon to generate the necessary VCF and BAM files for tertiary analysis in Part I. The implementation of VSPipeline allowed for rapid import and project generation for a predefined cancer gene panel project template in… Read more »
In our last part of this series, we showed how to run a pre-built workflow template via VSPipeline to automatically import and filter sample variants to streamline the search for clinically relevant variants. Now, we can deep-dive into our filtered, pathogenic variants to fully understand and capture their final classification and interpretation. Filtered Germline Variants for ACMG Guidelines The VSPipeline… Read more »
VSPipeline: Automating your Tertiary Workflows The first part of this “Automating & Standardizing your NGS Workflow” blog series covered the secondary analysis steps of read alignment and variant calling with Sentieon. The next step is to transition into the tertiary analysis via utilization of our workflow automation tool, VSPipeline. VSPipeline operates as a command-line tool meant to simplify the deployment… Read more »
Secondary Analysis with Sentieon: Rapid and Accurate Variant Calling This blog post will cover the utilization of secondary analysis tools to produce a list of high-quality variants and associated coverage data. This data will serve as the main, importable content for the tertiary stage of analysis where variants are interpreted and classified for their impact on a patients’ disorder. We… Read more »
As our regular customers may know, we write our blogs to provide relevant content to any NGS-based analysis with VarSeq. Our goal here at Golden Helix is to provide top quality software and guidance on how to use the software efficiently to perform variant analysis. However, this blog series will take a more general perspective and supply some insight into… Read more »
When using VarSeq; annotations, application settings, and assessment catalogs are all stored locally. Sometimes these resources can grow to large space grabbing directories, causing you to either purchase additional storage devices or getting rid of previously downloaded resources you might need down the road. But there’s hope! You can set where you want all of your data stored to be… Read more »
Smoothing Hurdles into Speed Bumps when creating Annotation Sources Although most researchers assume that getting the pile of VCF sequence files is the largest hurdle in moving towards an analysis, there still exists the looming step of normalizing the variant calls in annotation sources to make variant comparison easier. In this ever-refining field of study, VarSeq continually works to increase… Read more »
Clinical Variant Analysis for Cancer – Applying AMP Guidelines to Analyze Somatic Variants Detecting cancer at an early stage can make it much more treatable. Developing tests and making them clinically actionable is crucial to beat this disease. This eBook covers the state-of-the-art gene panel tests for cancer. Of course, there is more that can be done. The field is… Read more »
Clinical Variant Analysis for Cancer – Applying AMP Guidelines to Analyze Somatic Variants Somatic variants can manifest in different ways: There is a difference between the interpretation of germline and somatic variants. The former is exclusively focused on establishing the level of pathogenicity vis a vis a particular disease. In contrast to that, when we assess the clinical implication of… Read more »
Clinical Variant Analysis for Cancer – Applying AMP Guidelines to Analyze Somatic Variants As described in my eBook “Genetic Testing for Cancer,” any bioinformatic pipeline for cancer ultimately calls variants based on the aligned reads that the sequencer generated. Variant calling is the process of reviewing a sequence alignment, typically in the form of a BAM file, to identify loci… Read more »